|
Action for first mild reaction:
- Check labels & recipient ID
- If IV infusion, slow transfusion
- Consider giving medication:
- Antipyretic for pyrexia, e.g. paracetamol
- Antihistamine for urticaria
- Hydrocortisone - not usually needed
- If IV infusion, continue transfusion at a slower rate with increased monitoring, e.g. BP/P/T 15-30 mins
- If symptoms increase, treat as a moderate or severe reaction
- Send adverse reaction notification form to Blood Bank.
- Samples and the bottles of the product are not required for mild reactions
but will be tested if you would like to send them.
Top
Action if a moderate or severe reaction is suspected:
- If IV infusion, stop transfusion and review
- Check label and recipient ID information is correct
- If IV infusion, replace IV set; give saline to keep vein open and, or maintain BP
- Call for medical assessment
- Obtain specimens:
- FBC and Serum biochemistry
- And consider need for:
- Blood gases if respiratory distress present
- Urine to check for haemoglobinuria
- Coagulation screen if bleeding
- Blood cultures if sepsis suspected
- Send
Notification Of Suspected Adverse Reaction To A Fractionated Blood Product form, bottles of the product with IV set attached (in plastic bag) to
Blood Bank and specimens to relevant labs.
- Notify Blood Bank by phone: discuss urgency of follow up tests and further transfusion needs.
- Discuss with NZBS Transfusion Medicine Specialist if severe reaction present
- Further treatment - depends on cause:
FOR ANY SEVERE TRANSFUSION REACTION:
Contact the On-call Transfusion Medicine Specialist, Haematologist or Blood Bank immediately. Contact details are here.
More Info
|
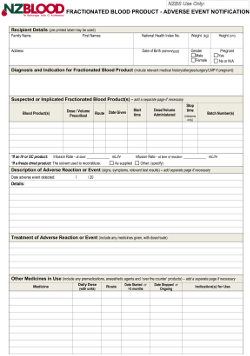
Notification Of Suspected Adverse Reaction To A Fractionated Blood Product form
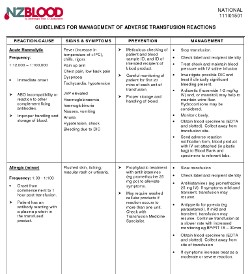
Guidelines For Management Of Adverse Transfusion Reactions
|

