|
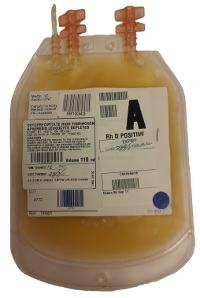
Presentation
|
- 80-120mL (contains a minimum of 0.4g/unit, average ~0.8g/unit fibrinogen)
|
|
|
ABO & RhD Compatibility
|
ABO
- Providing the product does not contain an ABO haemolysin (strong anti A or B antibody),
Cryoprecipitate may be given without regard to ABO type. Where an
ABO haemolysin (lysin) is present it may only be given to a
recipient with a compatible ABO group (see plasma compatible
donor group table above). This will be indicated on the label of the
cryoprecipitate as "For ABO Identical Recipient Only".
Rh(D)
- No anti-D immunoglobulin need be given if Rh(D) negative patients receive Rh(D) positive FFP or cryoprecipitate.
- Although frozen plasma components may contain small amounts of red cell stroma, sensitisation following transfusion of Rh(D) positive units is most unlikely, as stroma is less immunogenic than intact red cells.
Therefore Cryoprecipitate of any Rh(D) type may be given regardless of the Rh(D) type of the recipient. Contact Blood Bank if you are not sure.
|
|
Storage
|
- Once issued, Cryoprecipitate should be transfused as soon as possible.
- Once thawed, Cryoprecipitate may be stored at room temperature for up to 4 hours.
- Never store Cryoprecipitate in a fridge.
- If the transfusion cannot be started within 30 minutes, return Cryoprecipitate to Blood Bank immediately for appropriate storage.
|
|
Filter
|
- Use a standard blood infusion set which has a 170-200 micron
filter
- ANZSBT guidelines require that blood giving sets must be changed when transfusion is completed or every 12 hours if the transfusion episode is not yet complete. This is intended to reduce the risk of bacterial growth occurring.
- Any number of red cell units may be transfused during a 12-hour period provided the flow rate remains adequate. However specific manufacturer's recommendations defining the maximum number of units per blood administration set must not be exceeded.
- A new blood administration set should be used if infusion of another fluid, medication or platelets is to continue after the current transfusion. This is intended to reduce the risk of incompatible fluids or drugs causing haemolysis of residual red cells in the administration set or drip chamber.
- Please consult your DHB blood policy for further details.
- All fresh components, including cryoprecipitate, are leucodepleted
at source by NZBS. No bedside leucodepletion is necessary.
|
|
Pump
|
- Approved infusion pump devices may be used.
|
|
Rate and Duration
|
- Paediatrics:
- in a non-bleeding patient: infuse at 10-20mL/kg/hr
- in resuscitation: cryoprecipitate can be infused more rapidly based on the patient's haemodynamics
- Adults:
- in a non-bleeding patient: most adults will tolerate a unit of cryoprecipitate every 30 minutes.
- Consider a slower rate in patients with or at risk of congestive cardiac failure.
- Infusion of all components should be completed within 4 hours of leaving refrigerated storage.
|
Monitoring | |
|
DO NOT
|
- DO NOT add medication to Cryoprecipitate
- DO NOT use 5% Dextrose solutions (may induce
haemolysis)
- DO NOT use Lactated Ringer's or other balanced salt solutions that contain Calcium, as this may
induce clot formation in the blood bag and / or administration set.
|
|
Dose
|
- The standard therapeutic dose for cryoprecipitate is 1 unit per 30kg bodyweight. This provides enough fibrinogen to raise the fibrinogen level by 1g/L.
- For paediatric patients, a standard therapeutic dose is approximately 3mL/kg.
- For paediatric patients, the dose should be written in mL, not units.
|
|
Dose Calculator
|
|
|
More Info
|
|
|
